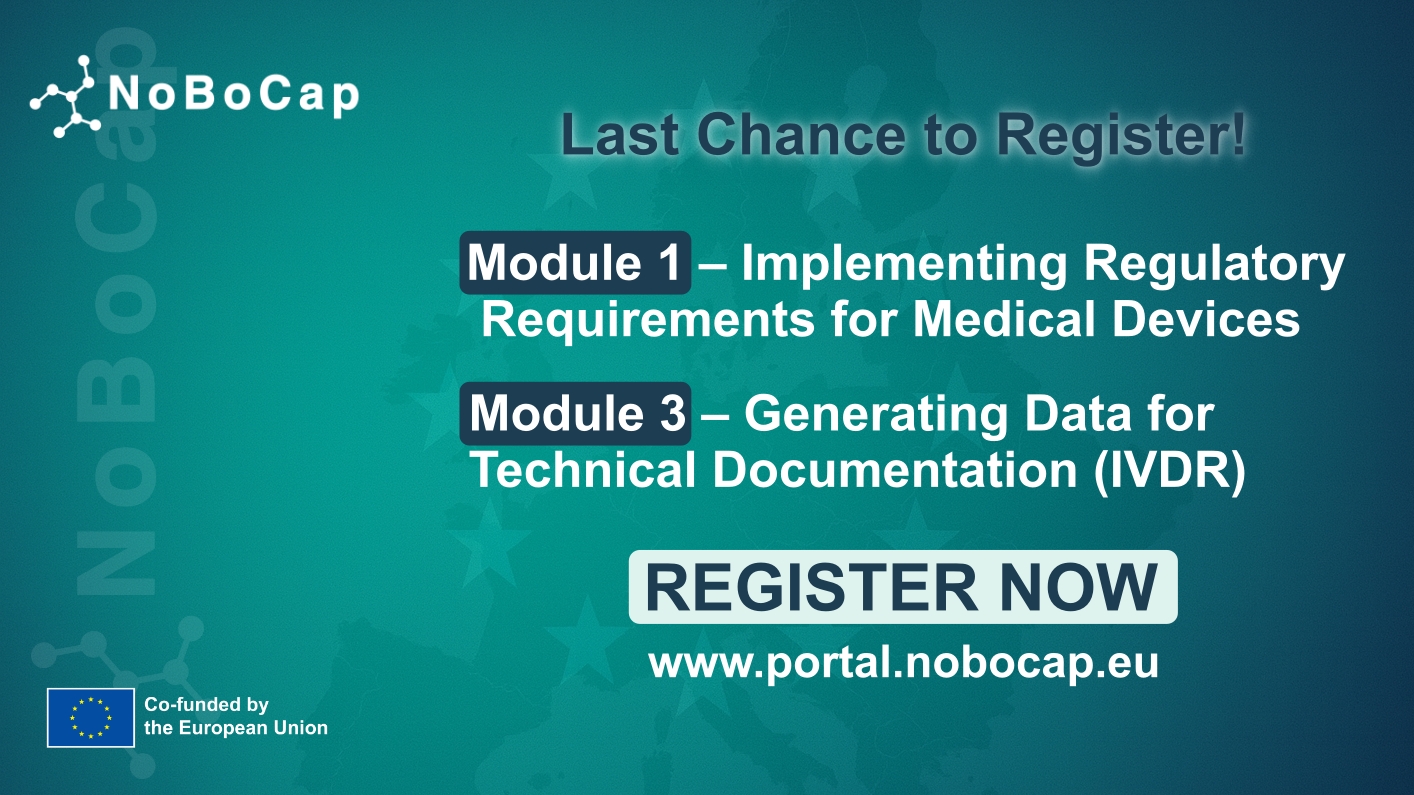


It was marked a pivotal moment in the NoBoCap initiative’s journey, as we hosted an interesting webinar dedicated to forging a dynamic community of innovators, united by a common goal: navigating the complexities of the EU MDR/IVDR Regulation. At the event over 30 participants, were eager to delve into the intricacies of this groundbreaking regulation.
Our attendees were treated to a presentation by Elizabeth Renzaglia from MEDVIA, who unveiled the vision and aspirations of the NoBoCap Community. This was followed by an engaging talk from Yves Verboven of EU4Health Solutions, who not only outlined the planned services of the community but also provided a roadmap for those aspiring to join this trailblazing initiative.
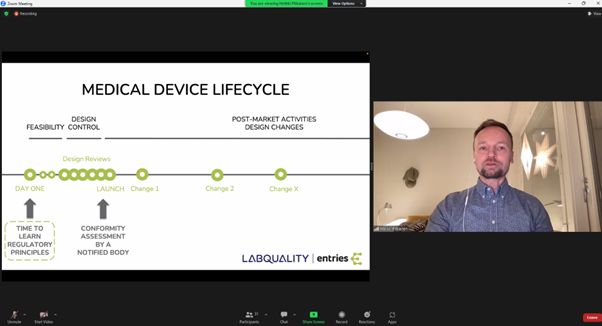
The webinar further raised the bar with Heikki Pitkänen from Lean Entries Ltd, who introduced an innovative e-tool designed to guide innovators through the new regulation, simplifying the process of identifying relevant codes and Notified Bodies.
But the learning didn’t stop there. Participants also gained insights into two significant DG SANTE initiatives through HaDEA, the Study supporting the monitoring of availability of medical devices on the EU market presented by Nina Zimmerman from Austrian National Public Health Institute GOG and the Study on Regulatory Governance and Innovation in the field of Medical Devices, presented by Daan Bijwaard from EYU. Big thanks to Stamatiki Kritas and Hellenic BioCluster (HBio) for hosting this event under the MEDIC NEST initiative.
So, if you re you a cluster or an innovation hub looking to be at the forefront of this exciting journey? Don’t miss your chance to be part of something revolutionary.
Apply now and join a community that’s redefining the future of medical innovation https://eu4healthsolutions.formstack.com/…/nobocap… to take your first step towards becoming a part of the NoBoCap Community.
Let’s innovate together!