The European Commission Health and Food Safety DG SANTE has just released two Guidance documents for both Manufacturers and Notified Bodies in the realm of Medical Devices (MDR) and In Vitro Diagnostic Medical Devices (IVDR):
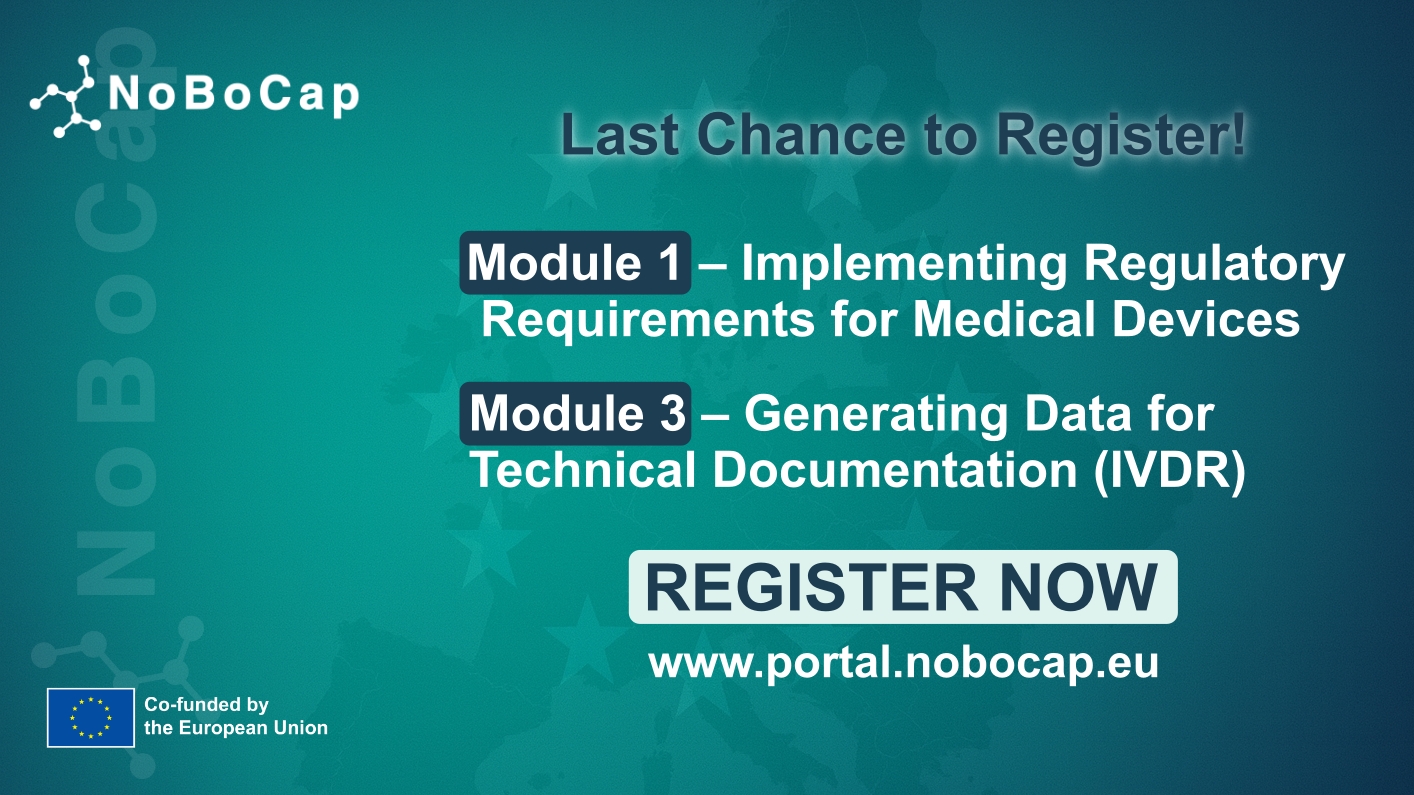
𝗠𝗗𝗖𝗚 𝟮𝟬𝟮𝟯-𝟱: 𝗚𝘂𝗶𝗱𝗮𝗻𝗰𝗲 𝗼𝗻 𝗾𝘂𝗮𝗹𝗶𝗳𝗶𝗰𝗮𝘁𝗶𝗼𝗻 𝗮𝗻𝗱 𝗰𝗹𝗮𝘀𝘀𝗶𝗳𝗶𝗰𝗮𝘁𝗶𝗼𝗻 𝗼𝗳 𝗔𝗻𝗻𝗲𝘅 𝗫𝗩𝗜 𝗽𝗿𝗼𝗱𝘂𝗰𝘁𝘀 – Dive into the specifics and insights tailored for manufacturers and notified bodies here: https://health.ec.europa.eu/system/files/2023-12/mdcg_2023-5_en.pdf
𝗠𝗗𝗖𝗚 𝟮𝟬𝟮𝟯-𝟲: 𝗚𝘂𝗶𝗱𝗮𝗻𝗰𝗲 𝗼𝗻 𝗱𝗲𝗺𝗼𝗻𝘀𝘁𝗿𝗮𝘁𝗶𝗼𝗻 𝗼𝗳 𝗲𝗾𝘂𝗶𝘃𝗮𝗹𝗲𝗻𝗰𝗲 𝗳𝗼𝗿 𝗔𝗻𝗻𝗲𝘅 𝗫𝗩𝗜 𝗽𝗿𝗼𝗱𝘂𝗰𝘁𝘀 – Get a comprehensive guide on demonstrating equivalence for Annex XVI products here: https://health.ec.europa.eu/system/files/2023-12/mdcg_2023-6_en.pdf
For more detailed information, you can access the full documents on the European Commission’s website: https://health.ec.europa.eu/medical-devices-sector/new-regulations/guidance-mdcg-endorsed-documents-and-other-guidance_en