MedTech Winter School: Online Registration Now Open
MedTech Winter School: Online Registration Now Open 26–28 January 2026 As of today, registrations for the MedTech Winter School: From Design to Clinical Evidence are accepted only for online participation.…
C-level Management Training
📣 The 4th Edition of the C-Level Training Short Course Starts this November!
Excellence Diploma for the Medical Innovation of the Year (Romanian Healthcare Awards 2025)
Excellence Diploma – Medical Innovation of the Year (Romanian Healthcare Awards 2025) We are proud to announce that the project “Notified Body Increased Capacity (NoBoCap)” was among the finalists of…
NoBoCap Panel at BEHEALTH 2025
NoBoCap Panel at BEHEALTH 2025: Medical Devices Regulations (MDR and IVDR) – Impacts on Healthcare Systems 📅 21 October 2025 | Bucharest & Online The NoBoCap Project will be featured…
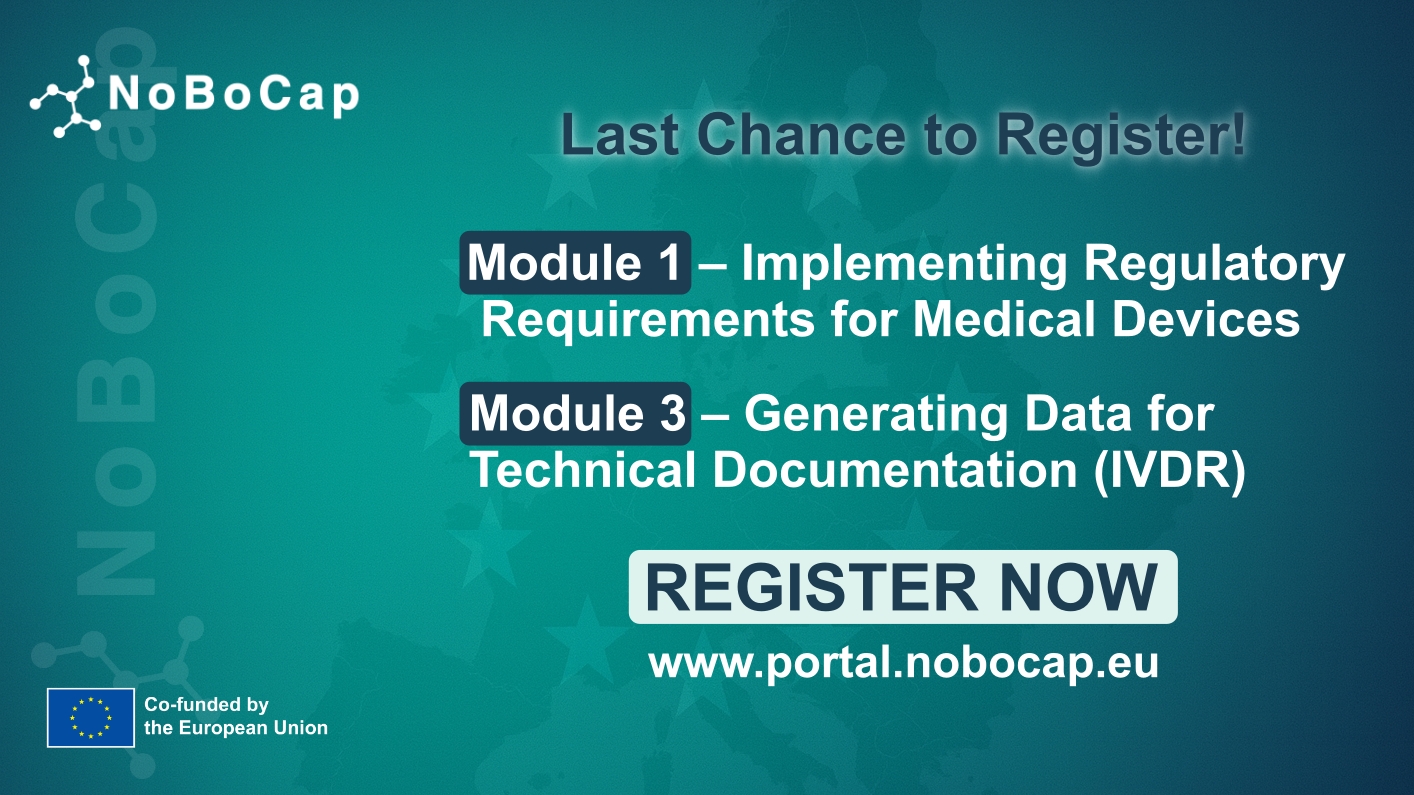
Last Chance to Register for the Final Training Modules
The NoBoCap project invites professionals across the MedTech sector to register for its final training modules in 2025. These expert-led programs are offered free of charge, providing a unique opportunity…
Module 2: Generating Data for Technical Documentation (MDR)
Need to navigate the complexities of the Medical Device Regulation (EU 2017/745)? This is your chance to gain the expertise needed to construct a Technical File and Clinical Evaluation Report…
C-level Management Training
📣 The 3rd Edition of the C-Level Training Short Course Starts this May!
Module 3: Generating Data for Technical Documentation (IVDR)
Are you ready to strengthen your expertise in Technical File construction and Performance Evaluation Report development under IVDR (EU 2017/746)? Module 3 – Generating Data for Technical Documentation (IVDR) begins…
Save the Date: Module 3
We’re just one month away from the start of Module 3 – Generating Data for Technical Documentation (IVDR), beginning on April 14th in collaboration with SGS and Technology University Dublin!…
Registration for Module 1 Now Closed!
The application process for Module 1, starting on February 10, 2025, is now officially closed. Don’t worry—your application will be considered for the next session, which is planned for Summer…
Module 1: Implementing Regulatory Requirements for Medical Devices
Unlock the skills you need to become an expert in Medical Devices Regulation (MDR) with our online postgraduate-level courses! Whether you’re aiming to work in medical device manufacturing, auditing, or…
Certification of Medical Devices in Lithuania: Challenges and Opportunities
The certification of medical devices and in vitro diagnostic medical devices is a critical factor for ensuring patient safety and regulatory compliance in the healthcare sector. As a member of the European Union (EU), Lithuania follows EU regulations and directives but lacks its own Notified Body to certify these devices.
Save the Date: Short Course on AI-supported Medical Devices
Are you ready to deepen your understanding of medical AI performance evaluation?
Short Course on Training for C-level Management (2)
Join us for an exclusive Short Course designed for C-level management professionals! Here’s a glimpse into what awaits you in our upcoming sessions: 8 November 2024 – Introduction session 15…
In Vitro Diagnostic Medical Device Regulation (IVDR) Postgraduate Training
Are you ready to take your understanding of In Vitro Diagnostic Medical Device Regulation (IVDR) to new heights?
SUMMIT BY NOBOCAP COMMUNITY
BRINGING TOGETHER EUROPETO UNLOCK MEDICAL DEVICE REGULATIONS & THE AI ACTFOR INNOVATORS AND SMES IN EUROPE 15-16 October 2025 Brussels, Belgium Download presentations Hear the speakers discuss building your knowledge…
Updates to MDCG 2022-4
On 27 May 2024, The Medical Device Coordination Group (MDCG) has released an update to the MDCG 2022-4 (Rev.2) guidance document.
Short Course on Training for C-level Management of MO’s on MDR/IVDR Implementation and Resource Allocation
Are you ready to navigate the complex world of EU Regulations in the Medical Device Industry?
Post-Webinar: Building a Community for Innovators to Unlock the EU Regulations
We’re thrilled to have hosted today an insightful event for the NoBoCap Community. At the event, we dove deep into the initiatives aimed at unlocking EU regulations for innovation. Our…
Medical Device (MDR) Postgraduate Training
Are you ready to take your understanding of Medical Device Regulation (MDR) to new heights?
Notified Bodies Survey on Certifications and Applications (MDR/IVDR) – updated document
The latest “Notified Bodies Survey on Certifications and Applications (MDR/IVDR)” is now available!
Medical Device (MDR) Postgraduate Training – Module 2
Are you ready to take your understanding of Medical Device Regulation (MDR) to new heights?
Survey: Regulatory governance and innovation in the field of medical devices and in vitro diagnostic medical devices
Dear Market Operators There is an important survey on the sectorial regulatory governance and innovation in the field of Medical Devices (MDR) and In Vitro Diagnostic Medical Devices (IVDR), conducted by The…
Guidance – MDCG endorsed documents
The European Commission Health and Food Safety DG SANTE has just released two Guidance documents for both Manufacturers and Notified Bodies in the realm of Medical Devices (MDR) and In Vitro Diagnostic Medical Devices (IVDR): 𝗠𝗗𝗖𝗚 𝟮𝟬𝟮𝟯-𝟱: 𝗚𝘂𝗶𝗱𝗮𝗻𝗰𝗲…
NoBoCap MDR Masters Level Module and Post-Program Evaluation
The NoBoCap project, recently completed the first cohort of its ambitious MDR (Medical Device Regulation) master’s Level Module 1 “Implementing Regulatory Requirements for Medical Devices” led by SGS (NB 1639) and TU Dublin.
CORE-MD Webinar | Providing high-risk medical devices for children – problems and proposals
Ready to embark on this knowledge-packed journey?
Clusters Meet Regions Conference, Iasi, Romania, 21-23 November 2023
Exciting News from the Clusters Meet Regions Conference in Iasi, Romania!
Brief Online Survey for Market Operators
We Need Your Insights for shaping the training curriculum developed under the NoBoCap Project! Participate in Our Brief Online Survey! Your voice is crucial. By taking a few minutes to…
Post-Webinar: Building a Community for Innovators to Unlock the EU Regulations
It was marked a pivotal moment in the NoBoCap initiative’s journey, as we hosted an interesting webinar dedicated to forging a dynamic community of innovators, united by a common goal:…
Notified Bodies Survey on certifications and applications (MDR/IVDR) – updated document
The Commission’s Directorate-General for Health and Food Safety has just released an important update for those closely following Medical Devices and In Vitro Diagnostic Medical Devices Regulations (MDR and IVDR). The “𝐍𝐨𝐭𝐢𝐟𝐢𝐞𝐝 𝐁𝐨𝐝𝐢𝐞𝐬…
FREE WEBINAR: Building a Community for Innovators to Unlock the EU MDR/IVDR Regulation
Join us in a webinar presenting the NoBoCap initiative on Building a Community for Innovators to Unlock the EU MDR/IVDR Regulation. NoBoCap is an EU co-funded project aiming to empower…
The Latest Version of the Manual on Borderline and Classification under MDR and IVDR is Here!
The latest updates in the world of medical device regulations in Europe.
The certification process and increased costs slow the growth of the medical devices market
Science and technology grow rapidly, and so does the supply of medical devices. It is estimated that there are more than 500 000 types of medical devices and in vitro diagnostic medical devices on the EU market.
Webinar: Regulation 2023/607 MDD Extension and its Impact on Manufacturers
Regulation 2023/607 was published in March 2023, extending MDD validity by up to 5 years. Join a free of charge webinar to learn about the impact on conformity assessment and marketing authorization of this regulation.
A new online MDR Postgraduate Course
A new online MDR Postgraduate Course will be organised starting with September 25, 2023 within NoBoCap project.
Notified Bodies Survey on certifications and applications (MDR/IVDR)
We are delighted to announce some significant developments from the European Commission.
Start-ups left hanging in EU medical device regulation snarl-up
Delays in getting the CE mark for medical devices are stretching the resources of European start-ups and forcing some to turn their attention to the US Europe is facing a…